电催化析氢是目前最有前途的绿色制氢技术之一,是实现可再生清洁能源的重要途径。但是电催化析氢反应的微观反应机制及其反应动力学的研究仍然存在一些瓶颈限制。
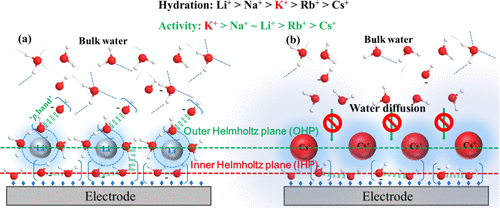
最近我们在电催化微观反应机制及其反应动力学方面的研究取得了一些最新的研究进展,提出了结构水(SWs)的概念,在分子水平上理解析氢反应对pH、碱金属离子结合水能力以及过渡金属羟基键合能力的依赖性。与过渡金属键合的结构水或者碱金属离子键合的结构水为析氢的催化活性中心。结构水一方面稳定水解离的过渡态(O-H键的活化);另一方面由于水中O原子p轨道在界面的重叠,形成了界面离域pi键,促进了电极表面的电子转移同时活化H,共同加速了析氢反应。
作为析氢催化活性中心的结构水(SWs)的概念与氢键水的概念完全不同,因为SWs是两个相邻的水分子,主要通过两个O原子的p轨道的空间重叠来结合,形成具有π键特征的局部化学键,由于空间轨道重叠,这为通过表面离域进行电子转移提供了一个替代通道。SWs作为协同电子和质子转移的替代通道,同时作为活化水O-H键的活性位点,这一概念完全不同于传统电催化剂弱相互作用对析氢、析氧以及O还原、CO2以及N2还原反应机制的理解。运用我们发展的界面态(PBIS)模型,我们成功的解释了电催化反应的一些异常反应现象,如无金属催化剂催化的反应机制,如碳点、杂原子掺杂的多孔碳材料的催化活性起源。
相关成果以activation of H2O tailored by interfacial electronic states at a nanoscale interface for enhanced electrocatalytic hydrogen evolution为题发表在《journal of the american chemical society au》(jacs au, 2022, doi: 10.1021/jacsau.2c00187)。全文链接有如下:https://pubs.acs.org/doi/10.1021/jacsau.2c00187
当前的研究结果完全不同于传统的认知,在这里分享一下,希望大家多提批评意见!实际上这也是我们课题组第一篇电催化相关的研究论文。
结构水的相关推文见科学网:水有颜色,会发光吗?(颠覆水无色无味液体的传统认知)
abstract:
despite the fundamental and practical significance of the hydrogen evolution reaction (her), the reaction kinetics at the molecular level are not well-understood, especially in basic media. here, with ZIF-67-derived co-based carbon frameworks (Co/NCS) as model catalysts, we systematically investigated the effects of different reaction parameters on the her kinetics and discovered that the her activity was directly dependent not on the type of nitrogen in the carbon framework but on the relative content of surface hydroxyl and water (OHad/H2O) adsorbed on co active sites embedded in carbon frameworks. when the ratio of the OH-/H2O was close to 1:1, the co/nc nanocatalyst showed the best reaction performance under the condition of high-ph electrolytes, e.g., an overpotential of only 232 mv at a current density of 10 mA cm-2 in the 1 m KOH electrolyte. we unambiguously identified that the structural water molecules (SWs) in the form of hydrous hydroxyl complexes absorbed on metal centers {OHad·H2O@M+} were catalytic active sites for the enhanced her, where M+ could be transition or alkaline metal cations. different from the traditional hydrogen bonding of water, the hydroxyl (hydroxide) groups and water molecules in the sws were mainly bonded together via the spatial interaction between the p orbitals of o atoms, exhibiting features of a delocalized π-bond with a metastable state. these newly formed surface bonds or transitory states could be new weak interactions that synergistically promote both interfacial electron transfer and the activation of water (dissociation of o╟h bonds) at the electrode surface, i.e., the formation of activated H adducts (H*). the capture of new surface states not only explains pH-, cation-, and transition-metal-dependent hydrogen evolution kinetics but also provides completely new insights into the understanding of other electrocatalytic reductions involving other small molecules, including CO2, O2, and N2.
全文pdf免费链接:https://pubs.acs.org/doi/pdf/10.1021/jacsau.2c00187
与结构水(SWs)相关的几篇核心文献:
(1)Yang, T.; Hu, X.; Shan, B.; Peng, B.; Zhou, J.; Zhang, K. Caged structural water molecules emit tunable brighter colors by topological excitation. Nanoscale 2021, 13 (35), 15058– 15066, 链接如下:https://pubs.rsc.org/en/content/articlelanding/2021/NR/D1NR02389F;
(2)Zhou, J.; Yang, T.; Peng, B.; Shan, B.; Ding, M.; Zhang, K. Structural Water Molecules Confined in Soft and Hard Nanocavities as Bright Color Emitters. ACS Phys. Chem. Au 2022, 2 (1), 47– 58, 链接如下:https://pubs.acs.org/doi/10.1021/acsphyschemau.1c00020;
(3)Yang, T.; Shan, B.; Huang, F.; Yang, S.; Peng, B.; Yuan, E.; Wu, P.; Zhang, K. P band intermediate state (PBIS) tailors photoluminescence emission at confined nanoscale interface. Commun. Chem. 2019, 2, 132, 链接如下:https://www.nature.com/articles/s42004-019-0233-1
转载本文请联系原作者获取授权,同时请注明本文来自张坤科学网博客。
链接地址:https://m.sciencenet.cn/blog-486147-1342515.html?mobile=1
收藏


