博文
[转载]Onasemnogene abeparvovec基因疗法治疗症状性婴儿型脊髓性肌萎缩症1型-part1
|||
Onasemnogene abeparvovec基因疗法治疗症状性婴儿型脊髓性肌萎缩症1型
Summary 摘要
Background Spinal muscular atrophy type 1 is a motor neuron disorder resulting in death or the need for permanent ventilation by age 2 years. We aimed to evaluate the safety and efficacy of onasemnogene abeparvovec (previously known as AVXS-101), a gene therapy delivering the survival motor neuron gene (SMN), in symptomatic patients (identified through clinical examination) with infantile-onset spinal muscular atrophy. 背景 1型脊髓性肌萎缩症是一种运动神经元疾病,会导致患者在2岁前死亡或需要永久通气。我们的目的是评估onasemnogene abeparvovec(以前称为AVXS-101)的安全性和有效性,这是一种提供存活运动神经元基因(SMN)的基因疗法,适用于有症状的婴儿型脊髓性肌萎缩症患者(通过临床检查确定)。
Methods STR1VE was an open-label, single-arm, single-dose, phase 3 trial done at 12 hospitals and universities in the 方法 STR1VE 是一项开放标签、单臂、单剂量的 3 期试验,在美国的 12 所医院和大学进行。
USA. Eligible patients had to be younger than 6 months and have spinal muscular atrophy with biallelic SMN1 mutations (deletion or point mutations) and one or two copies of SMN2. Patients received a one-time intravenous infusion of onasemnogene abeparvovec (1·1 × 10¹⁴ vector genomes per kg) for 30–60 min. During the outpatient follow-up, patients were assessed once per week, beginning at day 7 post-infusion for 4 weeks and then once per month until the end of the study (age 18 months or early termination). Coprimary efficacy outcomes were independent sitting for 30 s or longer (Bayley-III item 26) at the 18 month of age study visit and survival (absence of death or permanent ventilation) at age 14 months. Safety was assessed through evaluation of adverse events, concomitant medication usage, physical examinations, vital sign assessments, cardiac assessments, and laboratory evaluation. Primary efficacy endpoints for the intention-to-treat population were compared with untreated infants aged 6 months or younger (n=23) with spinal muscular atrophy type 1 (biallelic deletion of SMN1 and two copies of SMN2) from the Pediatric Neuromuscular Clinical Research (PNCR) dataset. This trial is registered with ClinicalTrials.gov, NCT03306277 (completed). 美国。符合条件的患者年龄必须小于6个月,并患有脊髓性肌萎缩症,同时伴有双拷贝SMN1突变(缺失或点突变)和一个或两个SMN2拷贝。患者一次性静脉输注onasemnogene abeparvovec(每公斤1-1×10¹⁴载体基因组)30-60分钟。在门诊随访期间,从输注后第7天开始,每周对患者进行一次评估,持续4周,然后每月评估一次,直至研究结束(18个月大或提前终止)。主要疗效指标为:18 个月大时独立坐立 30 秒或更长时间(Bayley-III 第 26 项),14 个月大时存活(无死亡或永久通气)。安全性通过评估不良事件、伴随药物使用、体格检查、生命体征评估、心脏评估和实验室评估进行评估。意向治疗人群的主要疗效终点与来自儿科神经肌肉临床研究(PNCR)数据集的6个月或更小的1型脊髓性肌萎缩症(SMN1和SMN2双拷贝缺失)未治疗婴儿(23人)进行比较。该试验已在 ClinicalTrials.gov 登记,编号为 NCT03306277(已完成)。
Findings From Oct 24, 2017, to Nov 12, 2019, 22 patients with spinal muscular atrophy type 1 were eligible and received onasemnogene abeparvovec. 13 (59%, 97·5% CI 36–100) of 22 patients achieved functional independent sitting for 30 s or longer at the 18 month of age study visit (vs 0 of 23 patients in the untreated PNCR cohort; p<0·0001). 20 patients (91%, 79–100]) survived free from permanent ventilation at age 14 months (vs 6 [26%], 8–44; p<0·0001 in the untreated PNCR cohort). All patients who received onasemnogene abeparvovec had at least one adverse event (most common was pyrexia). The most frequently reported serious adverse events were bronchiolitis, pneumonia, respiratory distress, and respiratory syncytial virus bronchiolitis. Three serious adverse events were related or possibly related to the treatment (two patients had elevated hepatic aminotransferases, and one had hydrocephalus). 研究结果 2017年10月24日至2019年11月12日,22名1型脊髓性肌萎缩症患者符合条件并接受了onasemnogene abeparvovec治疗。22名患者中有13名(59%,97-5% CI 36-100)在18个月的研究访问中实现了30秒或更长时间的功能性独立坐立(对比未接受PNCR治疗的23名患者中的0名;P<0-0001)。20 名患者(91%,79-100])在 14 个月大时摆脱了永久性通气而存活下来(未接受 PNCR 治疗的患者队列中有 6 人[26%],8-44 人;P<0-0001)。所有接受onasemnogene abeparvovec治疗的患者都至少出现过一次不良事件(最常见的是热病)。最常见的严重不良事件是支气管炎、肺炎、呼吸困难和呼吸道合胞病毒支气管炎。3 例严重不良事件与治疗有关或可能有关(2 例患者肝转氨酶升高,1 例患者脑积水)。
Interpretation Results from this multicentre trial build on findings from the phase 1 START study by showing safety and efficacy of commercial grade onasemnogene abeparvovec. Onasemnogene abeparvovec showed statistical superiority and clinically meaningful responses when compared with observations from the PNCR natural history cohort. The favourable benefit–risk profile shown in this study supports the use of onasemnogene abeparvovec for treatment of symptomatic patients with genetic or clinical characteristics predictive of infantile-onset spinal muscular atrophy type 1. 解读 这项多中心试验的结果以 START 1 期研究的结果为基础,显示了商业级 onasemnogene 阿贝帕维c 的安全性和有效性。与PNCR自然史队列的观察结果相比,onasemnogene abeparvovec显示出统计学上的优越性和有临床意义的反应。这项研究中显示出的有利的收益-风险特征支持使用onasemnogene abeparvovec来治疗具有婴儿型脊髓性肌萎缩症1型遗传或临床特征的无症状患者。
Participants 与会者
We planned to enrol up to 20 patients (appendix p 12). To be eligible, patients had to be younger than 6 months and have spinal muscular atrophy type 1. They could be either symptomatic or presymptomatic and they had to have a genotype associated with traditionally defined spinal muscular atrophy type 1 (a disabling mutation of SMN1 [deletion or predicted protein null] and one or two copies of SMN2). Patients had to have a swallowing evaluation test performed before administration of gene replacement therapy and be up to date on childhood vaccinations. Symptoms of infantile onset spinal muscular atrophy were identified through a clinical exam or medical history of the potential participant. 我们计划最多招募 20 名患者(附录第 12 页)。患者年龄必须小于 6 个月,且患有 1 型脊髓性肌萎缩症。他们既可以是有症状的患者,也可以是无症状的患者,而且必须具有与传统定义的 1 型脊髓性肌萎缩症相关的基因型(SMN1 的致残性突变[缺失或预测蛋白缺失]以及 SMN2 的一个或两个拷贝)。患者在接受基因替代疗法前必须进行吞咽评估测试,并接受最新的儿童疫苗接种。婴儿期脊髓性肌萎缩症的症状可通过临床检查或潜在参与者的病史来确定。
Study design 研究设计
STR1VE was an open-label, single-arm, single-dose, multicentre, phase 3 trial done at 12 centres (hospitals and universities) in the USA. The study was done in accordance with the Declaration of Helsinki, International Council for Harmonisation and Good Clinical Practice guidelines, and regulatory requirements, including those relating to informed consent and the protection of patients in bio- medical research. Written informed consent was obtained from the parents or legal guardians of all patients before study participation. The study protocol and informed consent form were approved by the institutional review boards at each site. The redacted study protocol is available at ClinicalTrials.gov. STR1VE 是一项开放标签、单臂、单剂量、多中心的 3 期试验,在美国的 12 个中心(医院和大学)进行。该研究符合《赫尔辛基宣言》、国际协调委员会和《良好临床实践指南》以及监管要求,包括与生物医学研究中的知情同意和患者保护相关的要求。所有患者在参与研究前均已获得其父母或法定监护人的书面知情同意。 研究方案和知情同意书已获得各研究机构审查委员会的批准。经编辑的研究方案可在 ClinicalTrials.gov 网站上查阅。
Procedures 程序
Onasemnogene abeparvovec was administered as a one-time intravenous infusion of 1·1 × 10¹⁴ vector genomes [vg]/kg for 30–60 min via a peripheral vein. Patients were admitted to the hospital 1 day before the infusion and monitored for 48 h post-infusion before discharge. Because elevated aminotransferases were reported in the START trial,16 all patients received prophylactic prednisolone (approximately 1 mg/kg per day), beginning 24 h before the infusion up to 30 days or more after the infusion. Prednisolone was tapered depending on liver function tests and the T-cell response (measured as spot-forming cells per 10⁶ peripheral blood mononuclear cells). During the outpatient follow-up, patients were assessed once per week, beginning at day 7 post-infusion for 4 weeks and then once per month until the end of the study (at age 18 months or early termination). Eligible patients could also enrol in an ongoing long-term follow-up study (NCT04042025). Onasemnogene abeparvovec通过外周静脉一次性静脉输注1-1×10¹⁴载体基因组[vg]/kg,持续30-60分钟。患者在输注前 1 天入院,输注后监测 48 小时后出院。由于 START 试验报告了转氨酶升高的情况16 ,所有患者都接受了预防性泼尼松龙治疗(每天约 1 毫克/千克),从输注前 24 小时开始,直至输注后 30 天或更长时间。泼尼松龙的用量根据肝功能检查和 T 细胞反应(以每 10 个外周血单核细胞中形成斑点的细胞数计算)的情况逐渐减少。在门诊随访期间,从输液后第 7 天开始,每周对患者进行一次评估,为期 4 周,然后每月评估一次,直至研究结束(18 个月大或提前终止)。符合条件的患者还可以参加正在进行的长期随访研究(NCT04042025)。
Outcomes 成果
The coprimary endpoints were the proportion of patients who achieved functional, independent sitting for 30 s or longer (Bayley-III item 26, “Child sits alone without support for at least 30 seconds”)19 at the 18 months of age study visit and survival at age 14 months. Survival was defined as absence of death or permanent ventilation (tracheostomy or ≥16 h daily non-invasive ventilation support for ≥14 days in the absence of acute reversible illness or perioperative ventilation). Coprimary efficacy endpoints were compared with a literature-based, histori- cal cohort4 of untreated infants with spinal muscular atrophy type 1 (Pediatric Neuromuscular Clinical Research Network [PNCR]; appendix pp 1,2,4,10), which enrolled patients between 2005 and 2009, with follow up for up to 36 months.4 This natural history population study4 consisted of patients with a biallelic deletion of functional SMN1 (common homozygous deletion) and two copies of SMN2, for whom enrolment data were available to evaluate survival.4 Efficacy was evaluated only in the intention-to-treat (ITT) population, defined as symptomatic patients with the most common spinal muscular atrophy type 1 genotype (biallelic deletion mutations of SMN1 [no functional copies] and two copies of SMN2 without the known gene modifier mutation (c.859G>C),who received an intravenous infusion of onasemnogene abeparvovec. 主要终点是在患者 18 个月大时,达到独立坐立 30 秒或更长时间(Bayley-III 第 26 项,"儿童在无支持的情况下独自坐立至少 30 秒")19 的患者比例,以及 14 个月大时的存活率。 存活定义为无死亡或永久通气(气管造口术或在无急性可逆性疾病或围术期通气的情况下,每天无创通气支持时间≥16 小时,且≥14 天)。 主要疗效终点与一项基于文献的、历史性队列研究4 (Pediatric Neuromuscular Clinical Research Network [PNCR];附录 pp 1,2,4,10)进行了比较,该队列研究的对象是未经治疗的 1 型脊髓性肌萎缩婴儿,研究时间为 2005 年至 2009 年,随访时间长达 36 个月。 这项自然史人群研究4 包括功能性 SMN1 双拷贝缺失(常见的同基因缺失)和 SMN2 双拷贝缺失的患者,这些患者的入组数据可用于评估存活率。 疗效仅在意向治疗(ITT)人群中进行评估,意向治疗人群是指接受onasemnogene abeparvovec静脉输注的最常见脊髓性肌萎缩症1型基因型(SMN1双拷贝缺失突变[无功能拷贝]和SMN2两个拷贝,无已知基因修饰突变(c.859G>C))的无症状患者。
The secondary outcomes were the proportion of patients who maintained the ability to thrive at age 18 months and the proportion of patients who were independent of ventilatory support at age 18 months based on data from the Trilogy 100 device (Philips, Cambridge, MA, USA). Ability to thrive is a composite endpoint defined by swallowing function, nutritional support, and weight maintenance. All three criteria were required to meet this composite endpoint, namely (1) the ability to tolerate thin liquids shown by a formal clinical swallowing assessment (eg, bedside swallow exam); (2) feeding exclusively by mouth, defined as not receiving nutrition through a feed- ing tube or other non-oral methods; and (3) maintaining weight greater than the third percentile for the appro- priate age and sex.20,21 Ventilatory support was defined as requiring no daily ventilator support or usage. Ventilator support due to acute reversible illness and perioperative ventilation were excluded from this definition. 次要结果是根据 Trilogy 100 设备(飞利浦公司,美国马萨诸塞州剑桥)的数据,18 个月大时保持茁壮成长能力的患者比例和 18 个月大时无需呼吸机支持的患者比例。茁壮成长能力是一个综合终点,由吞咽功能、营养支持和体重维持来定义。达到该综合终点需要满足所有三项标准,即:(1)通过正式的临床吞咽评估(如床旁吞咽检查)显示具有耐受稀薄液体的能力;(2)完全通过口腔进食,即不通过喂食管或其他非口腔方式接受营养;以及(3)体重维持在适当年龄和性别的第三百分位以上20,21。因急性可逆性疾病和围手术期通气导致的呼吸机支持不包括在此定义中。
The prespecified exploratory outcomes were: achieve- ment of developmental gross motor milestones (Bayley-III criteria),19 achievement of sitting independently for 10 s or more (per WHO criteria),21 age at which indepen- dent sitting (≥30 s) was first achieved, improvement of motor function (CHOP INTEND criteria7,22 or fine and gross motor domain components of the Bayley-III scales).19 Developmental milestones were assessed using independent, centrally reviewed video confirmation (Bayley-III scales) at anytimepoint during the study, up to and including age 18 months. All prespecified exploratory endpoints are reported except for compound motor action potential, which will be reported in a follow-up manuscript, because of the breadth and depth of these data ana- lyses. Safety was assessed through evaluation of adverse events, concomitant medication usage, physical examina- tions, vital signs assessments, cardiac assessments, and laboratory evaluations. 预设的探索性结果包括:达到粗大运动发育里程碑(Bayley-III 标准)19 、达到独立坐立 10 秒或以上(根据世界卫生组织标准)21 、首次达到独立坐立(≥30 秒)的年龄、运动功能改善(CHOP INTEND 标准7、22 或 Bayley-III 量表中精细和粗大运动领域的组成部分)。19 在研究过程中的任何时间点,包括 18 个月大之前(含 18 个月大),均采用独立、集中审核的视频确认(Bayley-III 量表)对发育里程碑进行评估。由于这些数据分析的广度和深度,除复合运动动作电位外,报告了所有预先指定的探索性终点,该终点将在后续手稿中报告。安全性通过不良事件评估、伴随药物使用、体格检查、生命体征评估、心脏评估和实验室评估进行评估。
Statistical analysis 统计分析
The redacted statistical analysis plan is available at ClinicalTrials.gov. In the PNCR4 cohort (n=23) used for comparison with our STR1VE cohort, no patient achieved sitting without support (≥30 s) and the survival rate at age 14 months was 26% (six of 23 patients). As a substitute for comparison against a rate of zero, we assumed that at most, no more than 0·1% of untreated patients with spinal muscular atrophy type 1 attain sitting without support (≥30 s) at age 18 months and 26% survive at age 14 months, on the basis of observations from the matched PNCR dataset.4 This current STR1VE study was calculated to have more than 90% power with α=0·025 to detect a significant difference in independent sitting using a one-sided exact binomial test and more than 80% power with α=0·05 to detect a significant difference in survival using a two-sided Fisher’s exact test, on the basis of enrolment of 15 patients into the ITT population, assumptions per matched PNCR dataset,4 and preliminary results from START.16 The two primary efficacy endpoints were assessed in sequence: independent sitting was assessed first and survival was assessed only if the independent sitting assessment met statistical significance. The two secondary endpoints were assessed in sequence: ability to thrive was assessed first and ventilatory support was assessed second. Again, as a substitute for comparison against a rate of zero, it was assumed that at most, no more than 0·1% of untreated patients with spinal muscular atrophy type 1 attain these secondary outcomes at age 18 months. These outcomes were analysed using one-sided exact binomial tests. For comparison, 95% CIs were estimated using two-sided tests and 97·5% CIs using one-sided tests. Safety data are descriptive, and no comparative analyses were done. Data were analysed using SAS software (version 9.4). This study is registered with ClinicalTrials.gov, NCT03306277 (completed). 经编辑的统计分析计划可在 ClinicalTrials.gov 网站上查阅。 在用于与我们的 STR1VE 队列进行比较的 PNCR4 队列(人数=23)中,没有患者在没有支撑的情况下实现坐立(≥30 秒),14 个月大时的存活率为 26%(23 名患者中的 6 名)。作为与零存活率进行比较的替代方法,我们根据匹配的 PNCR 数据集的观察结果,假定未经治疗的 1 型脊髓性肌萎缩症患者中最多只有 0-1% 的患者在 18 个月大时能实现无支撑坐起(≥30 秒),26% 的患者在 14 个月大时存活。经计算,目前的 STR1VE 研究在 α=0-025 时具有超过 90% 的力量,可通过单侧精确二项式检验检测出独立坐位的显著差异;在 α=0-05 时具有超过 80% 的力量,可通过双侧费雪精确检验检测出存活率的显著差异。 两个主要疗效终点按顺序进行评估:首先评估独立坐姿,只有当独立坐姿评估达到统计学意义时才评估生存率。两个次要终点按顺序评估:首先评估茁壮成长能力,其次评估呼吸支持能力。同样,作为与零比率进行比较的替代方法,假定未经治疗的 1 型脊髓性肌萎缩症患者在 18 个月大时达到这些次要终点的比例最多不超过 0-1%。这些结果采用单侧精确二项检验进行分析。为了进行比较,使用双侧检验估算 95% CI,使用单侧检验估算 97-5% CI。 安全性数据为描述性数据,未进行比较分析。数据使用 SAS 软件(9.4 版)进行分析。本研究已在 ClinicalTrials.gov 登记,编号为 NCT03306277(已完成)。
Results 成果
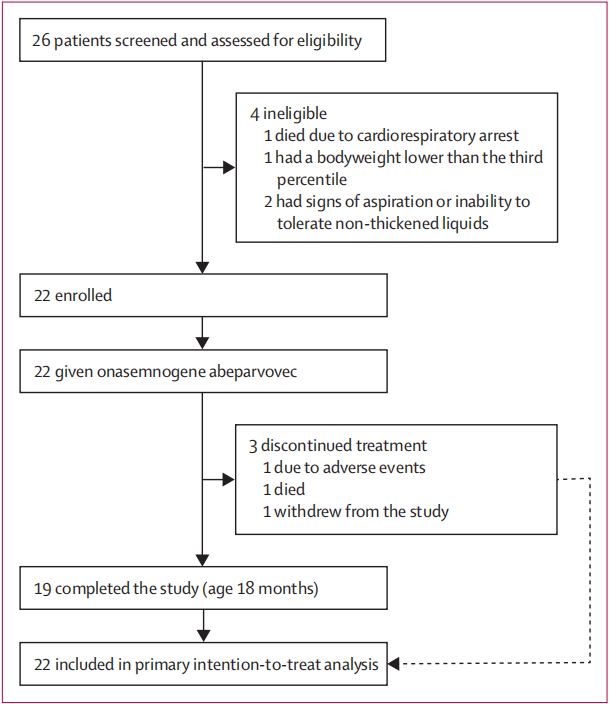
From Oct 24, 2017, to Nov 12, 2019, 26 infants with spinal muscular atrophy were screened and assessed for eligibility. 22 patients were eligible and given onasemno- gene abeparvovec (figure 1). Four patients were excluded during screening. The protocol called for 20 patients to be enrolled; however, when recruitment was stopped at 20 patients, two additional patients were undergoing screening, so they were included in the study. Although patients with one copy of SMN2 were eligible, none were enrolled. 25 patients were tested for anti-AAV9 antibodies during screening (none had exclusionary titres >1:50). No presymptomatic patients were enrolled, although the protocol allowed their inclusion. 2017年10月24日至2019年11月12日,26名患有脊髓性肌萎缩症的婴儿接受了筛查和资格评估。22名患者符合条件,并接受了asemno-gene abeparvovec治疗(图1)。在筛选过程中,有四名患者被排除在外。方案要求招募 20 名患者,但在招募到 20 名患者时,又有两名患者正在接受筛查,因此他们也被纳入了研究。虽然有一个 SMN2 拷贝的患者符合条件,但没有一人被纳入研究。25 名患者在筛查过程中接受了抗 AAV9 抗体检测(没有人的滴度超过 1:50)。虽然方案允许纳入无症状患者,但没有纳入这些患者。
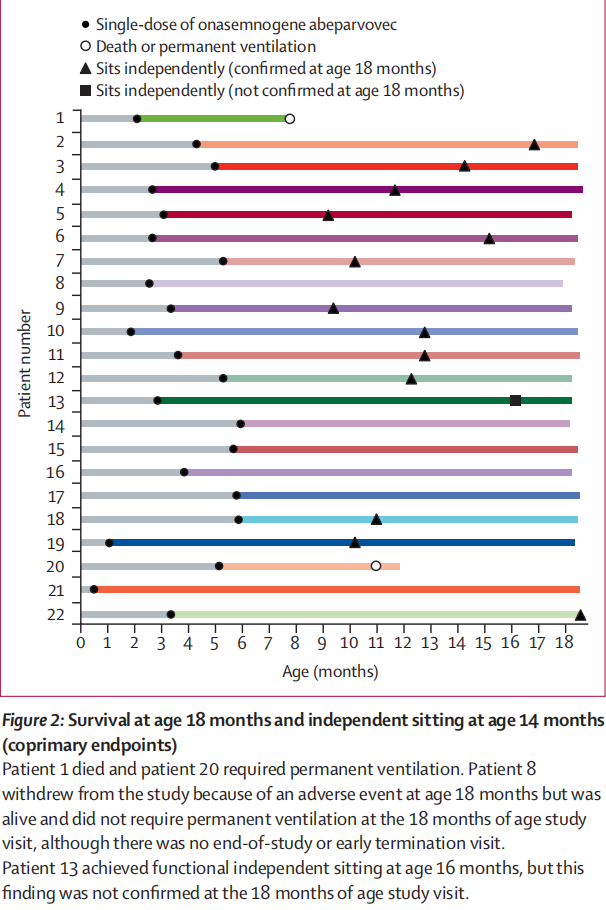
Clinical signs and symptoms ranged widely, as reflected in the range of CHOP INTEND scores (18–54),from less symptomatic (leg hypotonia and patella areflexia) to more symptomatic (profound hypotonia, global areflexia, and weakness). Patients were not excluded for muscle weakness or hypotonia, but rather if they had evidence of bulbar weakness or respiratory impairment as shown by the swallowing test and oxygen saturation during sleep. All enrolled patients met the enrolment criteria (ITT population), were symptomatic, were able to swallow thin liquids at baseline, and did not require any non-oral feeding or ventilatory support. Baseline characteristics are shown in table 1. The mean age at onasemnogene abeparvovec dosing was 3·7 months (SD 1·6). 19 (86%) of 22 patients completed the study. Two of three patients discontinued treatment before age 14 months (the 26% survival mark; figure 2): one patient died at age 7·8 months because of respiratory failure (considered unrelated to onasemnogene abeparvovec) and one patient’sparents or guardians withdrew consent at age 11·9 months after the patient met the definition of requiring permanent non- invasive ventilation at age 11·0 months. Another patient withdrew because of a serious adverse event (respiratory distress) at age 18·0 months; however, this patient was alive and did not require permanent ventilation and, therefore, was included as having survived without permanent ventilation at age 18 months. 临床症状和体征的范围很广,这反映在 CHOP INTEND 评分(18-54 分)的范围上,从症状较轻(腿肌张力低下和髌骨屈曲)到症状较重(极度肌张力低下、全身屈曲和无力)不等。 患者不因肌无力或肌张力减退而被排除在外,但如吞咽测试和睡眠时血氧饱和度显示患者存在球部肌无力或呼吸障碍,则应排除在外。所有入组患者均符合入组标准(ITT人群),无症状,基线时能吞咽稀薄液体,不需要任何非口喂养或呼吸支持。 基线特征见表 1。服用onasemnogene abeparvovec时的平均年龄为3-7个月(SD 1-6)。22 名患者中有 19 名(86%)完成了研究。三名患者中有两名在 14 个月大前(26% 的存活率标志;图 2)中断了治疗:一名患者在 7-8 个月大时因呼吸衰竭死亡(被认为与 onasemnogene abeparvovec 无关),一名患者在 11-9 个月大时,其父母或监护人在患者 11-0 个月大时符合需要永久性非侵入性通气的定义后撤回了同意。另一名患者在 18 个月大时因发生严重不良事件(呼吸窘迫)而撤回同意书;但该患者仍存活且不需要永久通气,因此被列为在 18 个月大时无需永久通气而存活的患者。
13 (59%, 97·5% CI 36–100) of 22 patients achieved the coprimary endpoint of functional independent sitting for 30 s or longer at the 18 months of age study visit (vs 0 of 23 untreated patients in the PNCR cohort4; p<0·0001; figure 2). An additional patient achieved this milestone at age 16·0 months, but was uncooperative at the 18-month visit; as a result, this patient was not judged to have achieved the primary endpoint. For the 14 patients who achieved independent sitting, this developmental milestone was achieved at a median age of 12·6 months (IQR 10·2–15·2). 20 of 22 patients (91%, 95% CI 79–100) survived without requirement of permanent ventilation at age 14 months, compared with six of 23 patients in the PNCR natural history cohort (26%, 8–44; p<0·0001). 22 名患者中有 13 名(59%,97-5% CI 36-100)在 18 个月大时达到了功能性独立坐立 30 秒或更长时间的共同主要终点(与 PNCR 队列中 23 名未接受治疗的患者中的 0 名相比4;P<0-0001;图 2)。另有一名患者在 16-0 个月大时达到了这一里程碑,但在 18 个月大时不合作;因此,这名患者未被判定达到主要终点。在实现独立坐立的 14 名患者中,实现这一发育里程碑的中位年龄为 12-6 个月(IQR 10-2-15-2)。22 例患者中有 20 例(91%,95% CI 79-100)在 14 个月大时无需永久通气即可存活,而 PNCR 自然病史队列的 23 例患者中有 6 例(26%,8-44;P<0-0001)。
At age 18 months, 18 (82%, 97·5% CI 59·7–100·0) patients did not use ventilatory support (secondary end- point; vs 0 of 23 patients in PNCR; p<0·0001).4 Overall, 15 (68%) patients did not use non-invasive ventilatory support at anytime during STR1VE. Of the seven patients who used non-invasive ventilation, five had documented previous use of Trilogy 100 and two had used other types of non-invasive ventilation. 18 个月大时,18 名患者(82%,97-5% CI 59-7-100-0)未使用通气支持(次要终点;与 PNCR 23 名患者中的 0 名相比;P<0-0001)。在使用无创通气的 7 名患者中,有 5 人曾使用过 Trilogy 100,2 人使用过其他类型的无创通气。
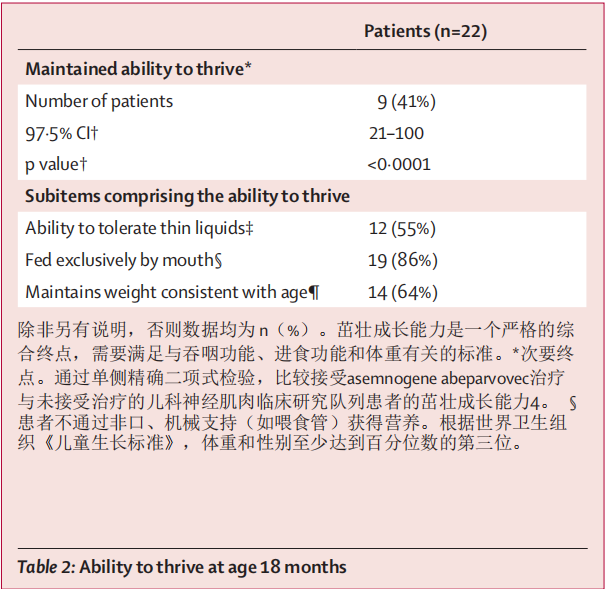
Nine (41%, 97·5% CI 21–100) patients maintained the ability to thrive (secondary endpoint; vs 0 patients in PNCR; table 2). 14 (64%) patients maintained a weight consistent with age at 18 months (table 2). Three patients (14%) used non-oral feeding support at age 18 months or at time of withdrawal from the study; two patients underwent gas- trostomy tube placements, and one was on feeding support (nasojejunal tube) at premature discontinuation (death). Four other patients received intermittent or transient non- oral feeding support (for 6–91 days) during the study but did not require non-oral feeding support at study end. Overall, 15 (68%) patients did not require feeding support at any point during the study. 12 (55%) patients had a normal or functional swallow result at age 18 months when tested forthin or very thin liquids. Seven (32%) other patients who had formal swallow tests at age 18 months had normal or functional swallow, but for consistencies other than thin or very thin liquids. All 19 patients with a formal swallowing test at the end of the study were feeding orally, with 17 having oral intake assessed at age 18 months; the 17 patients were all reported to be consuming 76–100% of their daily intake orally. 9名患者(41%,97-5% CI 21-100)保持了茁壮成长的能力(次要终点;与 PNCR 的 0 名患者相比;表 2)。14名患者(64%)在18个月时保持了与年龄相符的体重(表2)。3 名患者(14%)在 18 个月大或退出研究时使用了非口喂养支持;2 名患者接受了气管切开术,1 名患者在过早终止研究(死亡)时仍在使用喂养支持(鼻空肠管)。另有四名患者在研究期间接受了间歇性或短暂的非口喂养支持(6-91 天),但在研究结束时不需要非口喂养支持。总体而言,15 名患者(68%)在研究期间的任何时候都不需要喂养支持。12 名(55%)患者在 18 个月大时,在接受稀或非常稀的液体测试时,吞咽结果正常或功能正常。另外 7 名(32%)患者在 18 个月大时进行了正式的吞咽测试,其吞咽功能正常或正常,但测试的不是稀或非常稀的液体。所有 19 名在研究结束时接受过正规吞咽测试的患者都在进行口服喂养,其中 17 名患者在 18 个月大时接受了口服摄入量评估;据报告,这 17 名患者的口服摄入量均占其日常摄入量的 76%-100%。
19 (86%) patients achieved at least one motor mile- stone; other gross motor milestone achievements are shown in the appendix (p 5). Early and sustained improve- ment in CHOP INTEND assessments are shown in the appendix (pp 6, 7, 15), with mean increases from baseline of 6·9 points (SD 5·35) at 1 month post-dosing, 11·7 points (6·40) at 3 months, and 14·6 points (7·04) at 6 months. 21 (95%) patients achieved a CHOP INTEND score of 40·0 points or more, 14 (64%) achieved 50·0 points or more, and five (23%) achieved 60·0 points or more at any timepoint during the study. Children with spinal muscular atrophy type 1 almost never achieve or maintain CHOP INTEND scores greater than 40 points.4–6 Most patients showed a marked improvement in motor function performance, beginning as early as 1-month post-dosing, on the gross motor and fine motor subtests by age 18 months (appendix p 18). More patients were alive and free from permanent ventilation at 10·5 months in this study (21 [95%]) than in the PNCR cohort (50%; p<0·0001; figure 2,appendix p 13). 19名患者(86%)至少实现了一个运动里程碑;其他大运动里程碑的实现情况见附录(第5页)。CHOP INTEND 评估的早期和持续改善情况见附录(第 6、7 和 15 页),用药后 1 个月与基线相比平均增加 6-9 分(SD 5-35),3 个月时增加 11-7 分(6-40),6 个月时增加 14-6 分(7-04)。在研究期间的任何时间点,21 名患者(95%)的 CHOP INTEND 评分达到 40-0 分或以上,14 名患者(64%)达到 50-0 分或以上,5 名患者(23%)达到 60-0 分或以上。4-6 大多数患者的运动功能表现明显改善,最早在用药后 1 个月开始,到 18 个月大时在粗大运动和精细运动分项测试中均有所改善(附录第 18 页)。与 PNCR 队列(50%;P<0-0001;图 2,附录第 13 页)相比,本研究中有更多的患者(21 [95%])在 10-5 个月时仍存活且无需永久通气。
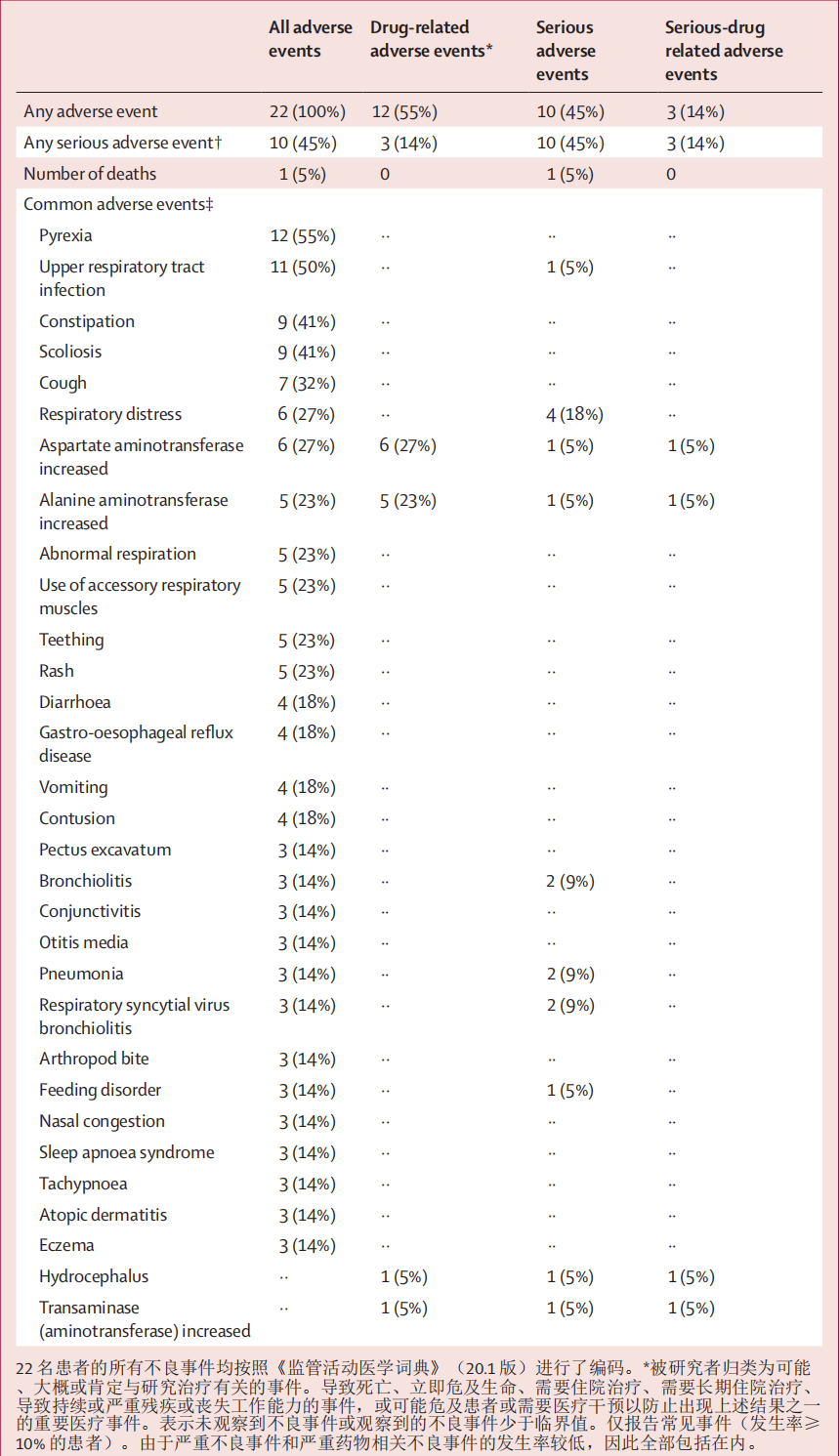
Two deaths were reported, both considered unrelated to treatment: one was due to respiratory distress and the other occurred during screening in a patient who was not enrolled or treated. All 22 enrolled patients had adverse events; however, three had serious adverse events con- sidered to be related to onasemnogene abeparvovec (two patients had elevated hepatic aminotransferases, and one had hydrocephalus and received ventriculoperitoneal shunt placement; table 3). The most frequently reported serious adverse events were bronchiolitis, pneumonia, respiratory distress, and respiratory syncytial virus bron- chiolitis, and were considered unrelated to onasemno- gene abeparvovec. The most common adverse event was pyrexia, which for two patients occurred within 1 week of dosing. For the other events there was no temporal association to the day of onasemnogene abeparvovec dosing. The mean latency to first episode of fever was 117·8 days (SD 109·71) and to any subsequent episode of fever was 200·2 days (125·38). Non-serious adverse events related to onasemnogene abeparvovec were mainly eleva- tions in liver aminotransferase concentration. 有两例死亡报告,均被认为与治疗无关:一例是由于呼吸窘迫,另一例发生在筛查期间,一名患者没有入组或接受治疗。所有 22 例入组患者均出现了不良反应;但有 3 例严重不良反应被认为与 onasemnogene abeparvovec 有关(2 例患者肝转氨酶升高,1 例患者出现脑积水并接受了脑室腹腔分流术;表 3)。最常报告的严重不良事件是支气管炎、肺炎、呼吸窘迫和呼吸道合胞病毒性支气管炎,这些不良事件被认为与onasemno-gene abeparvovec无关。 最常见的不良反应是脓毒症,其中两名患者在用药后一周内出现脓毒症。 其他不良反应与服用onasemno-gene abeparvovec的时间无关。首次发热的平均潜伏期为 117-8 天(标度 109-71),随后任何一次发热的平均潜伏期为 200-2 天(标度 125-38)。与onasemnogene abeparvovec有关的非严重不良事件主要是肝脏转氨酶浓度升高。
The protocol stipulated prophylactic prednisolone with an anticipated typical total dose of approximately 40 mg/kg × days if administration was not extended; patients received a cumulative dose ranging from 32·64 mg/kg × days to 254·25 mg/kg × days (median 44·6, IQR 41·5–50·25; mean 63·3, SD 61·62), with the overall duration ranging from 37 days to 203 days (median 62·5, 59·0–70·0; mean 73·7, 39·54). Seven patients had adverse events of liver aminotransferase elevations (alanine amino- transferase or aspartate aminotransferase; appendix p 8). The duration of prednisolone after onasemnogene abeparvovec treatment in these patients ranged from 58 days to 203 days, and there was no correlation noted between timing or magnitude of aminotransferase eleva- tions and prednisolone dosing. Additionally, 13 patients had elevated alanine aminotransferase, aspartate amino- transferase, or bilirubin, captured during scheduled laboratory evaluations and not reported as adverse events, with 12 of these occurring after dosing. Seven patients had an elevation in at least one of these serum chemistry values before dosing, but after enrolment. 方案规定,如果不延长给药时间,预防性泼尼松龙的预计总剂量约为 40 毫克/千克×天;患者接受的累积剂量从 32-64 毫克/千克×天到 254-25 毫克/千克×天不等(中位数 44-6,IQR 41-5-50-25;平均值 63-3,SD 61-62),总疗程从 37 天到 203 天不等(中位数 62-5,59-0-70-0;平均值 73-7,39-54)。七名患者出现肝脏转氨酶升高的不良反应(丙氨酸氨基转移酶或天冬氨酸氨基转移酶;附录 P 8)。这些患者在onasemnogene abeparvovec治疗后使用泼尼松龙的时间从58天到203天不等,没有发现转氨酶升高的时间或程度与泼尼松龙用药之间有任何关联。此外,13 名患者的丙氨酸氨基转移酶、天门冬氨酸氨基转移酶或胆红素升高,均在预定的实验室评估中发现,未作为不良事件报告,其中 12 例发生在用药后。有 7 名患者在用药前但在用药后出现至少一项血清化学值升高。
Symptoms of special interest were tracked in four broad categories: hepatotoxicity, haematological, cardiovascular, and neurological (appendix p 8). 13 (59%) patients had at least one event within the four categories (appendix p 8). Of the adverse events identified within the thrombo- cytopenia search of the laboratory values, only one patient had a platelet count of less than 75 × 10⁹ cells per L, with a nadir of 67 × 10⁹ cells per L on day 9 after dosing. One patient also had a platelet count of less than 75 × 10⁹ cells per L which was not reported as an adverse event. No events of thrombocytopenia were associated with clinical signs or symptoms of bleeding. None of the cardiovascular adverse events were interpreted as clinical signs or symptoms of cardiac pathology. None of the events were associated with clinically meaningful abnormalities on an electrocardiogram or echocardiogram. No cardiac thrombi were reported. None of the neurologi- cal events were suggestive of dorsal root ganglion abnormalities. 特别关注的症状分为四大类:肝中毒、血液病、心血管病和神经病(附录 p.8)。13(59%)名患者在这四个类别中至少出现了一种症状(附录第 8 页)。在血栓-细胞减少症实验室值搜索中发现的不良事件中,只有一名患者的血小板计数低于 75 × 10⁹个/升,用药后第 9 天的最低值为 67 × 10⁹个/升。还有一名患者的血小板计数低于每升 75 × 10⁹个细胞,但未作为不良事件报告。没有血小板减少事件与出血的临床症状或体征有关。没有一起心血管不良事件被解释为心脏病变的临床症状或体征。这些不良事件均与心电图或超声心动图上有临床意义的异常有关。无心脏血栓报告。所有神经系统不良事件均未提示背根神经节异常。
No patient had persistent vital sign abnormalities; all were transient and resolved. In some cases, vital signs normalised after treatment of the underlying cause (eg, tachycardia associated with fever). Atransient increase in hepatic aminotransferases occurred in many patients during the first month after treatment (not reported as adverse events). By the final study visit, all patients had been successfully tapered off of steroids and had normal aminotransferase concentrations. A transient decrease in platelets occurred in most patients at or around day 7 after dosing and returned to baseline concentrations by day 14 (appendix p 9). Two patients had decreases in platelet values that met laboratory criteria for thrombocytopenia (platelet count <75 × 10⁹ cells per L; grade 2). No patients had adverse events of bleeding (appendix p 8) and platelet counts returned to normal without a change in the prednisolone regimen. Left-ventricular ejection fraction for each patient was normal at final visit and no intra- cardiac thrombi were noted in echocardiogram results for any patient (appendix p 8). There was no clear or consistent increase in either the AAV9 or SMN cellular response (appendix p 3). 没有患者出现持续的生命体征异常;所有异常都是一过性的,并已缓解。在某些情况下,生命体征在病因治疗后恢复正常(如发热引起的心动过速)。许多患者在治疗后的第一个月出现肝脏转氨酶短暂升高(未作为不良事件报告)。在最后一次研究考察时,所有患者都已成功停用类固醇,转氨酶浓度正常。大多数患者的血小板在用药后第 7 天左右出现短暂下降,到第 14 天恢复到基线浓度(附录第 9 页)。两名患者的血小板值下降达到血小板减少的实验室标准(血小板计数<75×10⁹细胞/升;2级)。没有患者出现出血不良事件(附录 p8),血小板计数恢复正常,泼尼松龙治疗方案未作任何改变。在最后一次就诊时,每位患者的左心室射血分数均正常,超声心动图结果也未发现任何患者的心内血栓(附录 p.8)。AAV9 或 SMN 细胞反应没有明显或持续的增加(附录第 3 页)。
Discussion 讨论
This phase 3 study (STR1VE) builds on the phase 1 study (START) by showing safety and efficacy of onasemnogene abeparvovec as a treatment for infants with symptomatic spinal muscular atrophy type 1. We showed a favourable benefit–risk profile of onasemnogene abeparvovec, which is important given the few treatment options available for spinal muscular atrophy. Beyond supportive care, there are three FDA-approved treatment options (including onasemnogene abeparvovec), all of which aim to enhance SMN protein expression. Nusinersen, an antisense oligo- nucleotide that inhibits SMN2 exon 7 splicing, was approved by the FDA for spinal muscular atrophy in December, 2016;23 treatment requires intrathecal injections of four loading doses followed by maintenance doses every 4 months throughout the lifetime of the patient. Risdiplam, an SMN2 splice modifier, is the first oral drug to be approved (August, 2020), and requires daily dosage throughout the lifetime of the patient.24 Onasemnogene abeparvovec (approved in May, 2019), an SMN gene ther- apy, is potentially advantageous because administration is as a one-time intravenous infusion.9 这项3期研究(STR1VE)是在1期研究(START)的基础上进行的,它显示了onasemnogene abeparvovec治疗有症状的1型脊髓性肌萎缩症婴儿的安全性和有效性。我们证明了onasemnogene abeparvovec具有良好的收益-风险特征,这一点非常重要,因为脊髓性肌萎缩症的治疗选择很少。除了支持性治疗外,美国食品及药物管理局(FDA)批准了三种治疗方案(包括onasemnogene abeparvovec),它们都旨在增强SMN蛋白的表达。Nusinersen是一种抑制SMN2第7外显子剪接的反义寡核苷酸,于2016年12月获得FDA批准用于脊髓性肌萎缩症的治疗;23 治疗需要鞘内注射4次负荷剂量,然后在患者的整个生命周期中每4个月注射一次维持剂量。Risdiplam是一种SMN2剪接修饰剂,是首个获得批准的口服药物(2020年8月),需要在患者的整个生命周期内每天服药。24 Onasemnogene abeparvovec(2019年5月获批)是一种SMN基因疗法,具有一次性静脉输注给药的潜在优势。
Clinical and genetic features of the population enrolled in this study are characteristic of infants with the most severe spinal muscular atrophy compared with the natural history cohort (PNCR study).4 In addition to better than expected survival, more than half of patients given onasemnogene abeparvovec reached the other primary endpoint of independent sitting at the 18 months of age study visit, most achieved improvements in motor function (CHOP INTEND score ≥40; prespecified exploratory endpoint; appendix p 15), and many achieved other prespecified exploratory motor milestones, which differs markedly from the results seen in natural history studies of spinal muscu- lar atrophy. Although these motor milestones were acquired at a later stage than in children unaffected by spinal muscular atrophy, improvement was evident throughout the study. STR1VE was, to our knowledge, the first trial in symptomatic patients with spinal muscular atrophy to incorporate ability to thrive as a composite secon- dary endpoint. We showed relatively preserved physical growth, as defined by greater achievement of the ability to thrive endpoint, in nine (41%) patients receiving onasemno- gene abeparvovec, whereas essentially zero untreated patients achieve this challenging endpoint. Throughout STR1VE, most infants showed long-term ability to swallow thin or very thin liquids, feed without non-oral support, and maintain weight above the third percentile. Although patients who received onasemnogene abeparvovec showed improvements in independent sitting for 30 s or longer and survival free from permanent ventilation that are unprec- edented in untreated patients with spinal muscular atrophy, management of the disease with standards of care26,27 remains important after gene therapy. 与自然史队列(PNCR 研究)相比,本研究入组人群的临床和遗传特征是最严重脊髓性肌萎缩症婴儿的特征。4 除了存活率高于预期外,一半以上接受onasemnogene abeparvovec治疗的患者在18个月大时达到了另一个主要终点--独立坐立,大多数患者的运动功能得到改善(CHOP INTEND评分≥40;预设探索性终点;附录P 15),许多患者达到了其他预设探索性运动里程碑,这与脊髓性肌萎缩自然史研究的结果明显不同。虽然与未受脊髓性肌萎缩影响的儿童相比,这些运动里程碑的获得时间较晚,但在整个研究过程中,他们的进步是显而易见的。据我们所知,STR1VE 是第一项针对有症状的脊髓性肌萎缩患者进行的试验,该试验将茁壮成长的能力作为综合第二终点。 我们发现,在接受onasemno-gene abeparvovec治疗的9名患者(41%)中,身体发育得到了相对的保护,即达到了更高的茁壮成长能力终点,而未接受治疗的患者中达到这一挑战性终点的人数几乎为零。在整个 STR1VE 治疗过程中,大多数婴儿都表现出了长期吞咽稀薄或非常稀薄的液体、在没有非口支持的情况下进食以及将体重维持在百分位数第三位以上的能力。虽然接受onasemnogene abeparvovec治疗的患者在独立坐立30秒或更长时间以及无需永久通气的存活率方面都有所改善,但这是未经治疗的脊髓性肌萎缩症患者所无法比拟的,因此在基因治疗后,按照标准护理26、27管理疾病仍然非常重要。
The efficacy and favourable benefit–risk profile observed in the phase 1 START study17 was also shown in this study, with a population that was larger (n=22 vs n=12) and more diverse (multicentre vs single site), and with scaled up production of onasemnogene abeparvovec, which was manufactured identically to the commercially available product. Although both studies enrolled similar popula- tions, patients in STR1VE did not have baseline respiratory and feeding difficulties, whereas in START,16,17 two of 12 patients required ventilatory support and five patients required nutritional support. In both STR1VE and START, the survival and achievement of motor milestones after onasemnogene abeparvovec administration was remark- able and is unparalleled in the natural history of spinal muscular atrophy.4–6 All 12 patients in START17 who received the therapeutic dose were alive 24 months after treatment, and 20 of 22 patients in our study survived to age 18 months. In START,17 nine patients could sit independently for 30 s or longer compared with 14 patients in STR1VE. The differences in baseline characteristics and follow-up time (to age 18 months for STR1VE vs 24 months post-dosing for START) might explain the differences in achieving the motor milestone of sitting independently at the end of each study. START 1 期研究17 中观察到的疗效和有利的效益-风险概况也在本研究中得到了证实,但本研究的受试者人数更多(22 人对 12 人)、更多样化(多中心对单点),并且扩大了 onasemnogene abeparvovec 的生产规模,其生产工艺与市售产品完全相同。虽然两项研究的入组人群相似,但 STR1VE 的患者没有呼吸和喂养方面的基本困难,而 START16,17 的 12 例患者中有 2 例需要呼吸支持,5 例需要营养支持。在 STR1VE 和 START 两项研究中,onasemnogene abeparvovec 给药后的存活率和运动里程碑的实现都非常显著,这在脊髓性肌萎缩症的自然病史中是绝无仅有的。 在 START17 中,有 9 名患者可以独立坐立 30 秒或更长时间,而在 STR1VE 中,有 14 名患者可以独立坐立 30 秒或更长时间。基线特征和随访时间的差异(STR1VE 的随访时间为 18 个月,START 的随访时间为用药后 24 个月)可能是导致两项研究结束时在达到独立坐立这一运动里程碑方面存在差异的原因。
The most common adverse events in STR1VE (transient elevations in liver aminotransferases) that were considered related to onasemnogene abeparvovec had been identified in the initial phase 1 START study16 and reported in other AAV gene therapy trials, discussed by Colella and colleagues.28 In STR1VE, aminotransferase concentrations were normalised in all patients using varying doses and durations of prednisolone. No clinical signs of permanent liver damage were observed. Two patients had elevated hepatic aminotransferases, and one had hydrocephalus, for which the mechanism is unclear. Although there was no analysis of the relationship between the cellular and humoral response and aminotransferase increase, a more complete evaluation of the hepatic response across all clinical experience with onasemnogene abeparvovec will be reported separately. STR1VE 中最常见的不良事件(肝脏转氨酶的短暂升高)被认为与 onasemnogene abeparvovec 有关,这些不良事件已在最初的 START 第一阶段研究中发现16 ,并在其他 AAV 基因治疗试验中报告,Colella 及其同事对此进行了讨论28。没有观察到永久性肝损伤的临床症状。两名患者肝脏转氨酶升高,一名患者出现脑积水,其机制尚不清楚。虽然没有对细胞和体液反应与转氨酶升高之间的关系进行分析,但将单独报告对onasemnogene abeparvovec所有临床经验中肝脏反应的更全面评估。
Patients with spinal muscular atrophy might be inherently predisposed to acute liver injury. A 2019 study29 suggested that patients with spinal muscular atrophy were at increased risk of dyslipidaemia and fatty liver, which could predispose to hepatotoxicity. Further research is needed to understand the clinical applicability of this predisposition. Nonetheless, in children with elevated baseline aminotransferase, hepatic evaluation should be considered to mitigate any further elevations and con- comitant use of medications with hepatotoxic adverse events should be avoided whenever possible. 脊髓性肌肉萎缩症患者可能本身就容易发生急性肝损伤。2019 年的一项研究29 表明,脊髓性肌肉萎缩症患者发生血脂异常和脂肪肝的风险增加,这可能导致肝毒性。 要了解这种易感性的临床适用性,还需要进一步的研究。 不过,对于基线转氨酶升高的儿童,应考虑进行肝功能评估,以减轻进一步升高,并尽可能避免同时使用具有肝毒性不良反应的药物。
We also observed transient platelet decreases, similar to those described in START, without any evidence of associated bleeding. Based on non-clinical findings, cardiac adverse events and neurological adverse events that might implicate dorsal root ganglion inflammation were evaluated; however, there was no evidence that these concerns occurred in this study. In STR1VE, no cases of intracardiac thrombosis were reported, and no reported cardiac adverse events were considered clinically meaning- ful. Electrocardiogram and echocardiogram studies did not indicate evidence of pathology. Although specific neurophysiological exams were not done to assess dorsal root ganglion function during this trial, reported adverse events did not reveal any clinical signs or symptoms suggestive of dorsal root ganglia-related pain or sensory abnormalities associated with ganglionopathy. 我们还观察到短暂的血小板下降,与 START 中描述的情况类似,但没有任何相关出血的证据。 根据非临床研究结果,我们评估了可能与背根神经节炎症有关的心脏不良事件和神经系统不良事件;但是,没有证据表明本研究中出现了这些问题。在 STR1VE 中,没有心内血栓形成病例的报告,也没有心脏不良事件的报告被认为具有临床意义。 心电图和超声心动图研究未显示病理证据。 虽然在该试验期间没有进行特定的神经生理学检查以评估背根神经节的功能,但报告的不良事件并未显示任何提示背根神经节相关疼痛或神经节病变相关感觉异常的临床症状或体征。
A limitation of this study is the single-arm, open-label design. Given the commercial licensing of an effective intrathecal antisense oligonucleotide therapy (nusinersin) for spinal muscular atrophy, a placebo-controlled study was unethical, but a single-arm study was justifiable. The selected historical cohort represents the best available population but differs from the population in STR1VE in some important metrics. All 23 matched patients from the PNCR dataset4 with two copies of SMN2 had onset of symptoms before age 6 months, with ten enrolled within 3 months of the diagnosis, and the remaining 13 patients had more chronic disease than the rest of the cohort (mean age at enrolment 29 months, range 2–171 months). This cohort represents a mixed population of patients diagnosed recently and later, which differs from the STR1VE population in which patients were younger at enrolment. Consistent with the greater number of patients with chronic spinal muscular atrophy in PNCR than in STR1VE, there was greater use of feeding and ventilator support at baseline in the control group. The difference between the STR1VE group and the PNCR control group might thus underestimate the effect of onasemnogene abeparvovec that would be expected in a broad population of infants with spinal muscular atrophy. The degenerative course of untreated spinal muscular atrophy4,5 and favourable statistical comparison with the natural history cohort in the PNCR study4 provides confidence that our results are meaningful. Follow up in this study lasted until patients were aged 18 months, but optional further follow-up is ongoing (NCT04042025). 这项研究的局限性在于采用了单臂、开放标签设计。鉴于治疗脊髓性肌萎缩症的有效鞘内反义寡核苷酸疗法(nusinersin)已获得商业许可,进行安慰剂对照研究是不道德的,但进行单臂研究是合理的。所选的历史队列代表了现有的最佳人群,但在一些重要指标上与 STR1VE 的人群有所不同。PNCR数据集4中所有23名有两个SMN2拷贝的配对患者均在6个月前发病,其中10名患者在确诊后3个月内入组,其余13名患者比队列中的其他患者患有更多慢性疾病(入组时的平均年龄为29个月,范围为2-171个月)。该队列代表了新近确诊和较晚确诊患者的混合群体,这与 STR1VE 群体不同,STR1VE 群体中的患者在入组时年龄更小。 与 STR1VE 相比,PNCR 中的慢性脊髓性肌萎缩症患者人数更多,因此对照组基线时使用喂养和呼吸机支持的人数也更多。因此,STR1VE组与PNCR对照组之间的差异可能低估了onasemnogene abeparvovec对脊髓性肌萎缩症婴儿群体的影响。 未经治疗的脊髓性肌萎缩症的退化过程4,5 以及与 PNCR 研究中自然病史队列的有利统计比较4 使我们相信我们的结果是有意义的。本研究的随访持续到患者 18 个月大,但可选择的进一步随访仍在进行中(NCT04042025)。
The exploratory CHOP INTEND results in STR1VE showed rapid and early benefits of onasemnogene abeparvovec, with many patients maintaining the ability to thrive and most able to swallow effectively at the end of the study. The adverse events and laboratory findings were consistent with the known safety profile and are manage- able by screening patients for any underlying hepatic deficiency before treatment and closely monitoring their responses to treatment while adjusting the prednisolone dose accordingly. Similarly, platelet counts should be monitored before and after dosing. Cardiac pathology was not reported; however, patients should be monitored in accordance with product labelling. A post-hoc analysis did not suggest that the dorsal root ganglion inflam- mation observed in non-human primates occurred in humans (Chand DH, unpublished), but detailed ongoing neurological exams are warranted in patients after receiving onasemnogene abeparvovec. STR1VE 的探索性 CHOP INTEND 结果显示,onasemnogene abeparvovec 能迅速、早期获益,许多患者保持了茁壮成长的能力,大多数患者在研究结束时能有效吞咽。不良事件和实验室结果与已知的安全性特征相符,在治疗前筛查患者是否存在潜在的肝功能缺陷,并密切监测他们对治疗的反应,同时相应调整泼尼松龙的剂量,就能控制不良事件和实验室结果。 同样,用药前后也应监测血小板计数。没有关于心脏病理的报告,但应根据产品标签对患者进行监测。一项事后分析表明,在非人灵长类动物身上观察到的背根神经节炎症并未在人类身上发生(Chand DH,未发表),但患者在接受onasemnogene abeparvovec治疗后,应持续进行详细的神经系统检查。
https://m.sciencenet.cn/blog-3426442-1434972.html
上一篇:[转载]治疗 SMA 罕见病的孤儿药 Onasemnogene Abeparvovec (Zolgensma ® ) 的真实世界
下一篇:[转载]Onasemnogene abeparvovec基因疗法治疗症状性婴儿型脊髓性肌萎缩症1型-part2