博文
[转载]Hepatology:张琪/许燕/葛缅团队揭示糖异生代谢酶PCK1上调保护肝脏缺血-再灌注损伤的新机制
||
肝脏缺血-再灌注 (Ischemia-reperfusion, I/R) 损伤常伴发于肝脏切除、肝脏移植等各类肝脏外科手术,是导致肝脏外科手术术后肝功不全甚至肝功衰竭的主要危险因素之一[1]。
肝脏作为人体最大的实质性器官和消化器官, 在维持机体能量代谢中发挥着重要作用。在糖代谢方面, 肝脏参与糖原分解、合成以及糖异生等过程以维持血糖水平。在禁食状态下,肝脏通过分解糖原为机体提供能量,但在长时间禁食后糖异生产生的葡萄糖是机体葡萄糖的主要来源[2]。糖异生能够将非糖小分子底物(如甘油、乳酸、丙酮酸和生糖氨基酸等)转变为葡萄糖,以维持机体葡萄糖水平和能量稳态。肝脏和肾脏是哺乳动物主要的糖异生器官。
肝脏移植缺血期,由于氧气和能量物质供应中断,肝脏中正常的氧化磷酸化受到抑制,葡萄糖被大量用于无氧糖酵解为细胞供能,ATP产生迅速减少,糖酵解产物乳酸不断累积[3],过量乳酸堆积对细胞造成损伤。既往研究表明,术中葡萄糖输注能够增加肝糖原沉积,改善肝脏移植预后[4-6]。由于糖异生在生成葡萄糖的同时还能消耗细胞中堆积的乳酸等代谢产物,因此肝脏在缺血再灌注时是否会代偿性启动糖异生途径,来减少肝细胞能量的损失,减少乳酸堆积,这个问题一直有待阐明。
近日,来自中山大学附属第三医院生物治疗中心的张琪/许燕教授团队和手术麻醉中心葛缅教授团队在国际学术期刊Hepatology上在线发表了题为“m6A-mediated gluconeogenic enzyme PCK1 upregulation protects against hepatic ischemia-reperfusion injury ” 的研究成果。该研究发现肝脏在经历缺血再灌注损伤过程中可通过m6A依赖的方式激活糖异生限速酶——磷酸烯醇丙酮酸羧激酶1(PCK1)的表达、启动糖异生,从而减轻肝脏缺血-再灌注损伤。
该研究发现经历肝脏缺血再灌注损伤的肝脏组织内糖原储存减少,伴随糖异生限速酶PCK1的表达水平显著上调。临床数据分析显示,供肝组织中PCK1的表达水平与移植后肝脏损伤程度呈负相关,说明高水平PCK1能够促进移植后肝脏功能的恢复。体内外实验验证发现,抑制PCK1酶活性可显著加重肝脏的肝脏缺血再灌注损伤,而过表达PCK1可有效缓解肝脏缺血再灌注损伤。进一步探索发现,PCK1可通过糖异生途径消耗肝脏缺血再灌注过程堆积的乳酸,生成葡萄糖来维持能量代谢,进而缓解肝细胞的缺血再灌注损伤。机制研究发现肝脏缺血再灌注过程中PCK1的表达升高受到METTL3介导的m6A甲基化修饰调控。干预肝细胞内METTL3的表达与抑制PCK1一样,可以显著降低肝脏糖异生水平、加重肝脏的缺血再灌注损伤。
该研究首次探索了糖异生关键酶PCK1在肝脏缺血再灌注损伤中的作用和相关机制,揭示了肝脏缺血再灌注过程中m6A修饰介导的PCK1上调通过启动糖异生途径即METTL3/m6A-PCK1-糖异生轴促进临床肝脏外科手术后的肝功能恢复的关键作用,为临床从表观遗传调控来缓解肝脏缺血再灌注损伤提供了新思路。
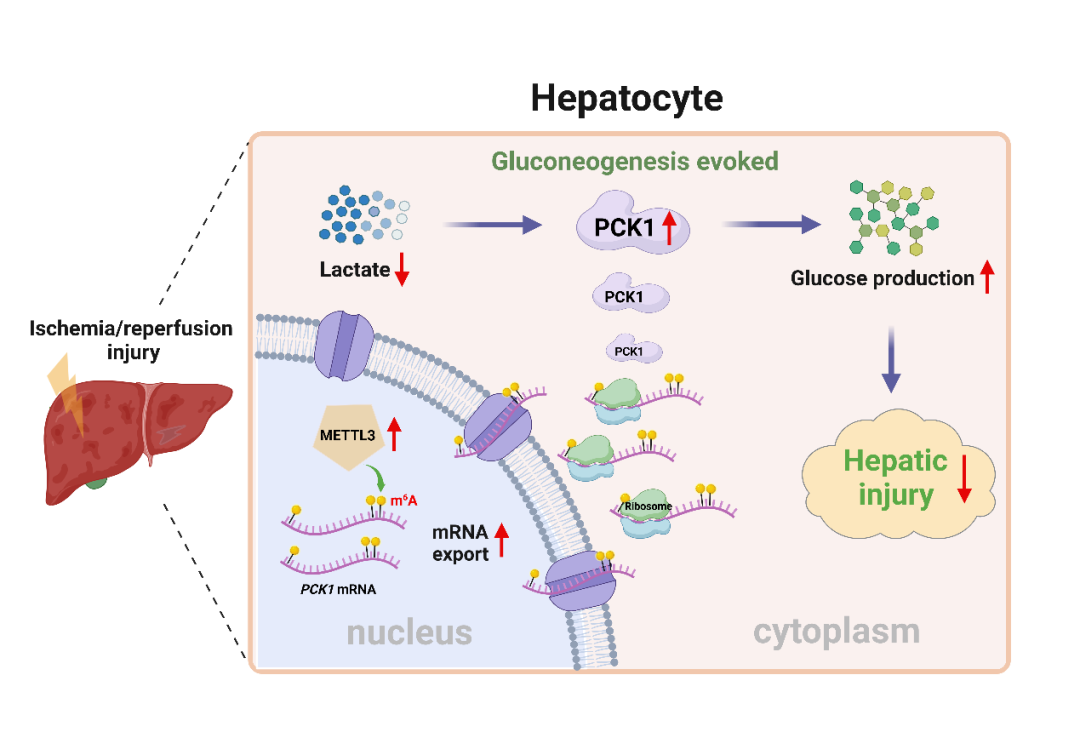
METTL3介导的m6A修饰上调PCK1表达保护肝脏I/R损伤的示意图
该研究通讯作者为中山大学附属第三医院生物治疗中心张琪教授、许燕副研究员、陈文捷副研究员和手术麻醉中心葛缅教授,第一作者为生物治疗中心博士研究生余珊珊、许燕副研究员和手术麻醉中心硕士研究生刘晓。
摘要:Abstract
Background & Aims: Ischemia-reperfusion (I/R) injury frequently occurs during liver surgery, representing a major reason for liver failure and graft dysfunction post-operation. The metabolic shift from oxidative phosphorylation to glycolysis during ischemia increased glucose consumption and accelerated lactate production. We speculate that donor livers will initiate gluconeogenesis, the reverse process of glycolysis in theory, to convert non-carbohydrate carbon substrates (including lactate) to glucose to reduce the loss of hepatocellular energy and foster glycogen storage for use in the early postoperative period, thus improving post-transplant graft function.
Approach & Results: By analyzing human liver specimens before and after hepatic I/R injury, we found that the rate-limiting enzyme of gluconeogenesis, PCK1, was significantly induced during liver I/R injury. Mouse models with liver I/R operation and hepatocytes treated with hypoxia/reoxygenation confirmed upregulation of PCK1 during I/R stimulation. Notably, high PCK1 level in human post-I/R liver specimens was closely correlated with better outcomes of liver transplantation. However, blocking gluconeogenesis with PCK1 inhibitor aggravated hepatic I/R injury by decreasing glucose level and deepening lactate accumulation, while overexpressing PCK1 did the opposite. Further mechanistic study showed that METTL3-mediated RNA N6-methyladinosine (m6A) modification contributes to PCK1 upregulation during hepatic I/R injury, and hepatic specific knockout of METTL3 deteriorates liver I/R injury through reducing the m6A deposition on PCK1 transcript and decreasing PCK1 mRNA export and expression level.
Conclusions: Our study found that activation of the METTL3/m6A-PCK1-gluconeogenesis axis is required to protect against hepatic I/R injury, providing potential intervention approaches for alleviating hepatic I/R injury during liver surgery.
DOI: https://doi.org/10.1097/HEP.0000000000000716
全文链接: https://journals.lww.com/hep/abstract/9900/m6a_mediated_gluconeogenic_enzyme_pck1.675.aspx
Yu, S., Liu, X., Xu, Y., Pan, L., Zhang, Y., Li, Y., Dong, S., Tu, D., Sun, Y., Zhang, Y., Zhou, Z., Liang, X., Huang, Y., Chu, J., Tu, S., Liu, C., Chen, H., Chen, W., Ge, M., & Zhang, Q. (2023). m6A-mediated gluconeogenic enzyme PCK1 upregulation protects against hepatic ischemia-reperfusion injury. Hepatology (Baltimore, Md.), 10.1097/HEP.0000000000000716. https://pubmed.ncbi.nlm.nih.gov/38085830/
参考文献:
1. Zhai Y, Petrowsky H, Hong JC, Busuttil RW, Kupiec-Weglinski JW. Ischaemia-reperfusion injury in liver transplantation-from bench to bedside. Nat Rev Gastroenterol Hepatol 2013;10:79-89.
2. Raddatz D, Ramadori G. Carbohydrate metabolism and the liver: actual aspects from physiology and disease. Z Gastroenterol 2007;45:51-62..
3. Han Y, Yuan M, Guo YS, Shen XY, Gao ZK, Bi X. Mechanism of Endoplasmic Reticulum Stress in Cerebral Ischemia. Front Cell Neurosci 2021;15:704334.
4. Boudjema K, Lindell SL, Belzer FO, Southard JH. Effects of method of preservation on functions of livers from fed and fasted rabbits. Cryobiology 1991;28:227-236.
5. Astarcioglu I, Adam R, Gigou M, Isaac J, Bismuth H. High levels of glycogen in the donor liver improve survival after liver transplantation in rats. Transplant Proc 1991;23:2465-2466.
6. Helton WS. Effect of intraportal glucose infusion on hepatic glycogen content and degradation, and outcome of liver transplantation. JPEN J Parenter Enteral Nutr 1993;17:192-193.
https://m.sciencenet.cn/blog-446272-1417220.html
上一篇:周末在嘉陵江畔跑了12.25公里
下一篇:[转载]肠道菌群代谢物通过Acetate-GPR43-NLRP3-MAVS-IFN-I信号轴抗流感病毒的分子机制