博文
首例氮转化为氨的铁基催化剂
||||
首例氮转化为氨的铁基催化剂
诸平
据物理学家组织网(Phys.org)2013年9月6日报道,美国加州理工学院(California Institute of Technology)的研究人员已经开发出了一种铁基催化剂,可以在溶液中使N2分子还原为氨(NH3)。此项研究结果2013年9月4日已经在《自然》(Nature)杂志网站发表——John S. Anderson, Jonathan Rittle & Jonas C. Peters. Catalyticconversion of nitrogen to ammonia by an iron model complex. Nature,501, 84–87. (05 September2013). doi:10.1038/nature12435.
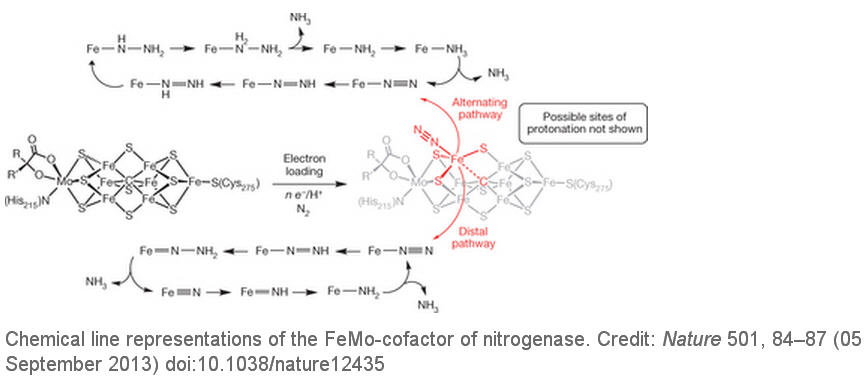
对于生命而言,将氮(N2)转换为氨(NH3)是一种必不可少的过程。尽管科学家对于化学模拟生物固氮的研究,从20世纪60年代就已经开始,主要有固氮酶活性中心模型化合物的研究,固氮酶催化机理研究分子氮配合物的研究等,经研究表明,固氮酶中心含有过渡金属与氮分子形成的分子氮配合物,这种配合物能使氮分子活化,易于被还原。科学家(无论是化学家还是生物学家)对于固氮酶的能力非常敬佩,一直想搞清楚某些植物的根瘤菌究竟是如何将空气中的氮分子转化为植物可以吸收利用的氮化合物。因为氮一般以含有键能较高的氮-氮三键的氮分子形式存在于自然界中,必须将这3个化学键完全破坏才能把该双原子分子中的2个氮原子分开。固氮酶实际上就是固氮作用中的催化剂,固氮酶使N2还原为NH3反应的活化能降低,从而使反应更容易进行。
科学家知道植物固氮过程的核心是酶的催化作用,而所有的酶都含有铁和其他金属原子,但人们无法解决在实际转换过程中能够发挥作用的金属原子的位置问题。科学家并没有在困难面前畏缩不前,而是知难而进。通过不断努力,加州理工学院的研究小组就已经构建了一个强有力的含铁催化剂,此催化剂能够将分子氮转化成氨。他们的成功标志着含铁催化剂的首次发现,而且可以使分子氮转化成氨的催化反应顺利进行。他们开发的催化剂有一个铁原子与一个配位体链接,而配位体的作用主要是针对氮分子。将氮分子还原为氨电子所做实际的工作是一种快速过程,只利用了电子和质子混合物中的一半。
JohnS. Anderson, JonathanRittle & JonasC. Peters三人小组的研究结果并没有解开固氮酶之谜,但他们也许提供了一个揭开固氮酶作用之谜的新思路。以前已开发的将氮分子转化为氨的方法,大多数依靠钼作为催化剂,而且需要高温高压。例如, 商业化的氨大规模生产,采用的是卡尔·博世(Carl Bosch)和弗里茨·哈伯(Fritz Haber)设计的方法。相比之下,加州理工学院的研究人员开发的氨合成工艺,不需要高温高压,在正常室温即可完成。研究人员欣然承认,他们的方法并不是要取代Haber–Bosch氨的生产工艺,其弱点就是不适合大规模生产的。因为每个催化剂分子只能转化7个氮分子就精疲力竭了。
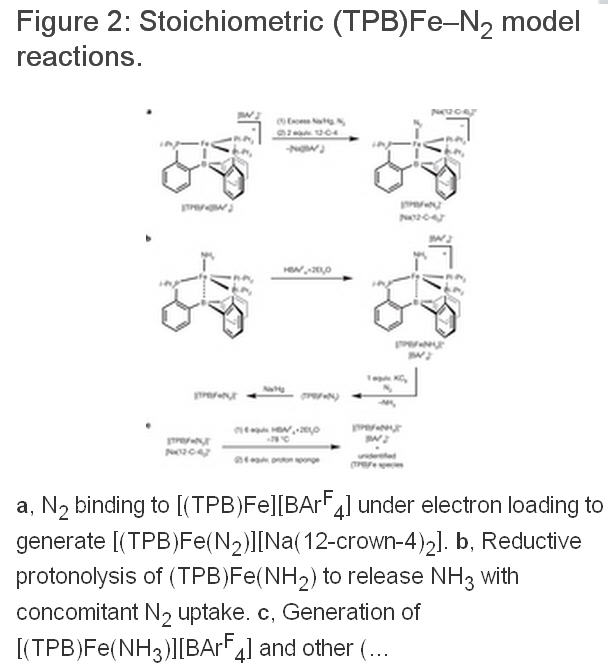
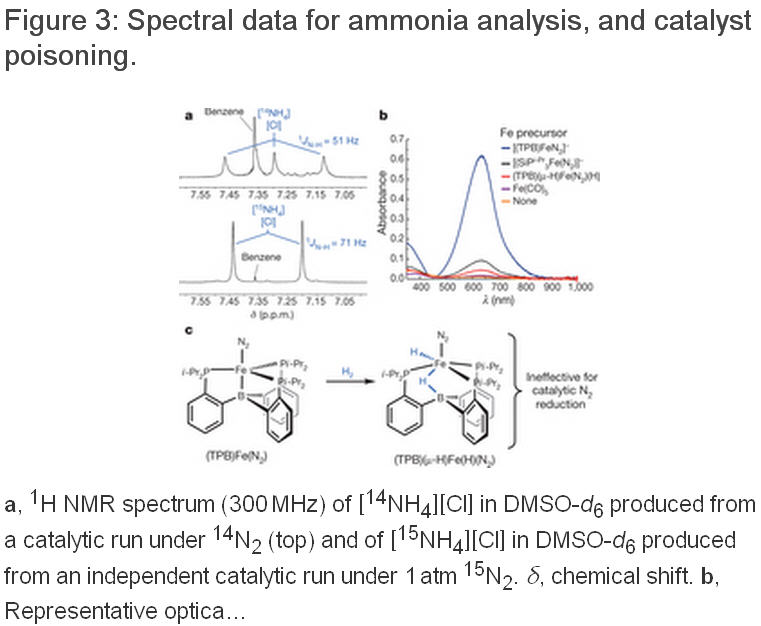
所有固氮酶都具有一个含铁和硫的辅因子(包括活性区域中的一个四聚体,例如FeMoCo)。在大多数固氮酶中,这个异金属拥有一个中央钼原子,而其他的则由钒原子或铁原子取代。虽然人们对于固氮酶的催化机理有所认识,但是对其具体的催化过程仍然有许多鲜为人知谜团。争论的核心要素一直在围绕着N2配位和还原的准确位置。在合成无机化学,早期阶段的重点是放在Mo上面,是因为Mo被认为是固氮酶的一种重要元素,因为已建立的Mo模型配合物可以显示出良好的调解作用,使N2按照化学计量关系转换为NH3。这种化学物质转换可以通过两种以Mo为中心的分子系统催化剂来完成。然而,现在人们认为铁是所有固氮酶中唯一必要的过渡金属,而最近的生物化学数据和光谱数据已经显示,在FeMo辅因子(FeMo-cofactor)中N2键合的位置以铁代替钼。加州理工学院的研究人员在文章中描述了一个三膦硼烷支撑的铁配合物,在温和条件下,可以催化使N2还原为NH3,而且在这种催化剂中,质子所占比例超过40%。研究人员的研究结果表明,单个铁的位置可以能够稳定催化形成氨过程中产生的各种NxHy中间体。铁的几何可调性是由铁和硼之间易弯曲的一种相互作用所给予的,而且似乎是有效的催化作用中最重要的。更多信息请浏览原文。
补充美国《化学与工程新闻》(C&EN)周刊的相关报道原文,供大家参考:
Issue Date: September 9, 2013
News Channels: Environmental SCENE, Biological SCENE
Keywords: nitrogen fixation, nitrogenase, Haber-Bosch

Conversion of dinitrogen to ammonia is a process that’s both essential to life on Earth and industrially important in fertilizer production. Researchers have long studied the enzymes that living things use to promote the reaction, but details of their catalytic mechanisms have remained elusive.
Now, Caltech scientists have created an iron complex that catalyzes this conversion in a manner that may help reveal the way nitrogenases do it. The iron catalyst and earlier molybdenum versions could also help lead to improved catalysts for reducing N2 to NH3 industrially.
Bacteria make nitrogenases and use them to convert N2 in air to NH3, a process called nitrogen fixation. The process is a primary source of nitrogen in proteins, nucleic acids, and other biomolecules. The Haber-Bosch process, which was developed in the early-20th century and uses a solid iron catalyst for adding H2 to N2 at high temperatures and pressures to form NH3 for fertilizer production, is now also a primary source of fixed nitrogen.
Most nitrogenases have an active-site cofactor—a molybdenum-iron cluster—but researchers don’t yet know whether it’s Mo or Fe that coordinates and reduces N2. In 2003, Richard R. Schrock’s group at MIT developed the first Mo complex that catalyzes N2 to NH3 conversion under mild conditions.Yoshiaki Nishibayashi of the University of Tokyo and coworkers reported another such Mo complex two years ago. Both Mo complexes are inefficient catalysts and break down quickly, but they work. That could not be said for any Fe-based complex, favoring the idea that nitrogenase’s Mo is the active metal.
John S. Anderson, Jonathan Rittle, and Jonas C. Peters at Caltech have now come up with the first Fe-based complex that directly catalyzes nitrogen fixation to NH3 under mild conditions (Nature 2013, DOI: 10.1038/nature12435).
The tris(phosphine)borane-supported Fe-N2 -complex’s catalytic activity is slightly lower than the Mo catalysts’, and it likewise breaks down quickly. But its supporting ligand “can be varied systematically to improve the catalyst, so there are good prospects for future improvement,” notes Patrick L. Holland of Yale University, who specializes in Fe-based cleaving reactions.
The Fe complex tips the balance of evidence back toward the idea that nitrogenase catalysis may be Fe-based. The study “provides favorable information for Fe claimants, but further study is necessary,” Nishibayashi says. One such claimant, Brian M. Hoffman of Northwestern University, says, “You can no longer use the argument that Mo must be it because it’s the only one shown to do the job.”
Peters and coworkers propose that a flexible Fe-boron interaction in their complex may play a key catalytic role and that a carbon-Fe interaction in nitrogenase may work similarly. “Through analogies like this, synthetic compounds can help chemists to gain insight into the mechanisms of enzymes,” Holland says. Nishibayashi also believes the result “provides useful and significant information to elucidate the reaction mechanism of the MoFe cofactor in nitrogenase.” But Schrock says, “It’s a stretch to say it tells us much, if anything, about the mechanism of reduction by various nitrogenases.”
The Fe-based and Mo-based complexes are possible first steps toward developing nitrogen fixation routes that are more economical than the energy-intensive Haber-Bosch process. “The work shows that chemistry just has a much wider range of elements and structures to choose from than nature had during evolution and that mechanistically there may be quite a few possibilities for efficient N2-fixing complexes,” says bioinorganic chemist Oliver Einsle of the University of Freiburg, in Germany.
https://m.sciencenet.cn/blog-212210-723077.html
上一篇:Liangfang Zhang(张良方)的几篇高引论文(修改稿)
下一篇:人尿代谢物发现3000余种并非最终结果