博文
WHO《实验室生物安全手册》第四版 Laboratory Biosafety Manual 4th edition
|
世界卫生组织(WHO)《实验室生物安全手册》(第四版)于2021年更新,该版本中生物安全的防范对象从“病原体和毒素”扩展至“生物因子”(biological agents),即“为防止无意接触生物因子或其意外释放而实施的控制原则、技术和做法”,其中,“生物因子”指有可能导致感染、过敏、毒性或以其他方式对人类、动物或植物造成危害的微生物、病毒、生物毒素、颗粒或其他传染性材料等。
The WHO Laboratory Biosafety Manual (LBM) has been in broad use at all levels of clinical and public health laboratories, and other biomedical sectors globally, serving as a de facto global standard that presents best practices and sets trends in biosafety.
LBM encouraged countries to accept and implement basic concepts in biological safety and to develop national codes of practice for the safe handling of biological agents in laboratories within their geographical borders.
This fourth edition of the manual builds on the risk assessment framework introduced in the third edition. A thorough, evidence-based and transparent assessment of the risks allows safety measures to be balanced with the actual risk of working with biological agents on a case-by-case basis.
This novel evidence- and risk-based approach will allow optimised resource use and sustainable laboratory biosafety and biosecurity policies and practices that are relevant to their individual circumstances and priorities, enabling equitable access to clinical and public health laboratory tests and biomedical research opportunities without compromising safety.
(1) 第四版PDF下载链接:https://www.who.int/publications/i/item/9789240011311
(2) Laboratory biosafety manual, 4th edition: Laboratory design and maintenance
https://www.who.int/publications/i/item/9789240011397
(3) Laboratory biosafety manual, 4th edition: Outbreak preparedness and resilience
https://www.who.int/publications/i/item/9789240011373
(4) Laboratory biosafety manual, 4th edition: Decontamination and waste management
https://www.who.int/publications/i/item/9789240011359
(5) Laboratory biosafety manual, 4th edition: Personal protective equipment
https://www.who.int/publications/i/item/9789240011410
中国CDC提供的下载地址:https://www.chinacdc.cn/lac/gzzd/gwfgbz/202003/t20200327_215579.htm
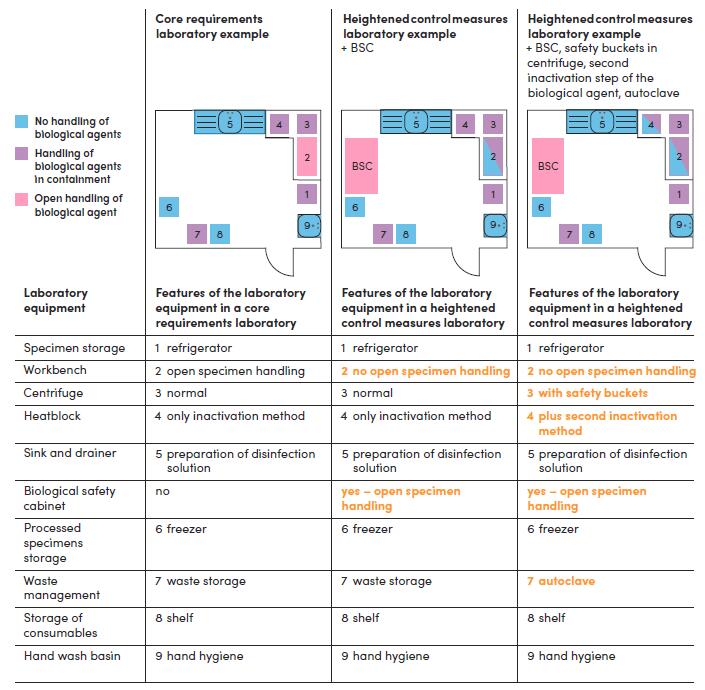
Core requirements Heightened control measures
Figure 7.2 Examples of workflow diagrams for laboratories with core requirements and heightened control measures as informed by the outcome of a risk assessment. These laboratories have similar laboratory activities but different risks. The core requirement laboratory works on biological agents that can be handled without containment. The laboratory with common heightened control measures includes a biological safety cabinet (BSC). The laboratory with additional heightened control measures for handling more hazardous infectious biological agents has a BSC, uses two inactivation methods, safety buckets in the centrifuge and waste inactivation by an autoclave. In the table below the workflow diagrams, the laboratory equipment needed for the core requirements is in black text, and the additional equipment for heightened control measures is in orange tex.
WHO第四版手册,将实验室生物安全防护要求分为“核心要求”、“加强要求”和“最高要求”,而不再用生物安全防护水平(BSL-1, 2, 3, 4)表示。

第4版《实验室生物安全手册》封面
《世界卫生组织(卫生组织)实验室生物安全手册》第一版于1983年由出版,《实验室生物安全手册》第二和第三版分别于1993年和2004年出版。《实验室生物安全手册》以前3个版本按风险、危险类别和生物安全防护级别对生物因子和实验室进行了分类。WHO认为,这种分法合乎逻辑,但也导致了一种误解,即生物因子的风险组直接对应于实验室的生物安全水平。事实上,针对一种特定情况的实际风险不仅受到所操作生物因子的影响,而且还受到所执行的程序和从事实验室活动的实验室人员能力的影响。因此第四版特别强调了基于风险评估和循证思维的理念,可以在每个个案基础上平衡安全措施与实际的风险,目的是使各国能够实施经济上可行和可持续发展的实验室生物安全以及与自身情况和优先事项相关的生物安全政策和措施。

《实验室生物安全手册》(第三版)中文版 下载链接:http://www.doc88.com/p-67434254538.html
https://www.docin.com/p-2893891259.html
https://m.sciencenet.cn/blog-446272-1359924.html
上一篇:综述:病毒与代谢 Viral Infections and Host Metabolism
下一篇:[转载]PERK1和糖基转移酶OGT调控糖异生关键酶FBP1的蛋白磷酸酶功能