博文
O-GlcNAc修饰增强Keratin 18蛋白与IDH之间的相互作用并促进胆管癌进展
|
胆管癌(cholangiocarcinoma, CCA)又称胆道癌,是一种高度异质性的胆道恶性肿瘤,约占原发性肝癌的15%和胃肠道恶性肿瘤的3%[1]。与大多数癌症一样,在 CCA 进展的复杂情况中,糖基化的改变,极大地影响了CCA的发生发展[2]。一些异常表达的聚糖和糖复合物(例如糖蛋白、糖脂)已被用作CCA诊断和预后预测的肿瘤标志物[3-5]。此外,某些独特的聚糖修饰也与CCA患者的短期生存相关,并在CCA细胞增殖、迁移、侵袭和化疗耐药等过程中发挥关键作用[6-8]。因此,针对CCA糖基化的功能阐明受到了越来越多的关注。
O-连接β-N-乙酰葡萄糖胺(O-linked β-N-acetyl-glucosamine, O-GlcNAc)修饰,是一类特殊的单糖O-糖基化修饰,仅发生在细胞质、细胞核及线粒体蛋白的丝氨酸或苏氨酸残基上,并且仅受单一的一对糖基转移酶(O‑GlcNAc transferase, OGT,催化O-GlcNAc糖基化)和糖基水解酶(O‑GlcNAcase, OGA,催化O-GlcNAc去糖基化)调控[9]。O-GlcNAc糖基化修饰的动态平衡对于维持细胞稳态至关重要,然而其在胆管癌进展中的机制还尚未清楚。目前针对O-GlcNAc修饰异常变化在胆管癌中的功能研究仍然有限,同时缺乏针对胆管癌系统性的蛋白质O-GlcNAc修饰的组学信息和机制探讨。
基于此,南京大学化学化工学院谢然课题组与国家蛋白质科学中心(北京)刘嘉琳副研究员、南京大学附属鼓楼医院消化内科王雷主任和南京大学化学化工学院马晶教授合作,围绕O-GlcNAc修饰的异常改变在胆管癌细胞中的功能展开研究,对胆管癌及胆管上皮细胞中蛋白质O-GlcNAc修饰进行了大规模组学鉴定,同时,探讨了O-GlcNAc修饰蛋白在胆管癌中的作用机制。近日,该研究发表在ACS Central Science期刊上,题为“O-GlcNAcylation Facilitates the Interaction between Keratin 18 and Isocitrate Dehydrogenases and Potentially Influencing Cholangiocarcinoma Progression”(DOI: 10.1021/acscentsci.4c00163);博士生孟祥凤为第一作者,南京大学附属鼓楼医院消化内科周悦为共一作者。该论文报道了关于胆管癌的化学糖蛋白质组学鉴定及糖基化功能研究的成果,作者基于非天然糖代谢标记技术辅助的完整糖肽组学鉴定方法(即Click-iG策略),对胆管癌及胆管上皮细胞中O-GlcNAc糖基化修饰的完整糖肽进行了组学鉴定及差异分析(图1),并关注到在胆管癌细胞中上调最为显著的角蛋白18(Keratin 18, K18)。
图1. 基于Click-iG策略对胆管癌及胆管上皮细胞中蛋白质O-GlcNAc修饰的组学鉴定
接下来,研究者进一步验证了Keratin 18(K18)蛋白中Ser30位点为主要的O-GlcNAc修饰位点。接着,我们构建了K18敲低及重组过表达K18-WT或K18-S30A的胆管癌稳转细胞株,并发现K18蛋白Ser30位点的O-GlcNAc修饰可能通过调控细胞周期检查点的蛋白表达,并增强K18蛋白的稳定性,从而在体内外水平促进胆管癌的细胞增殖(图2)。
图2.Keratin 18蛋白的O-GlcNAc修饰促进胆管癌细胞的体内外生长
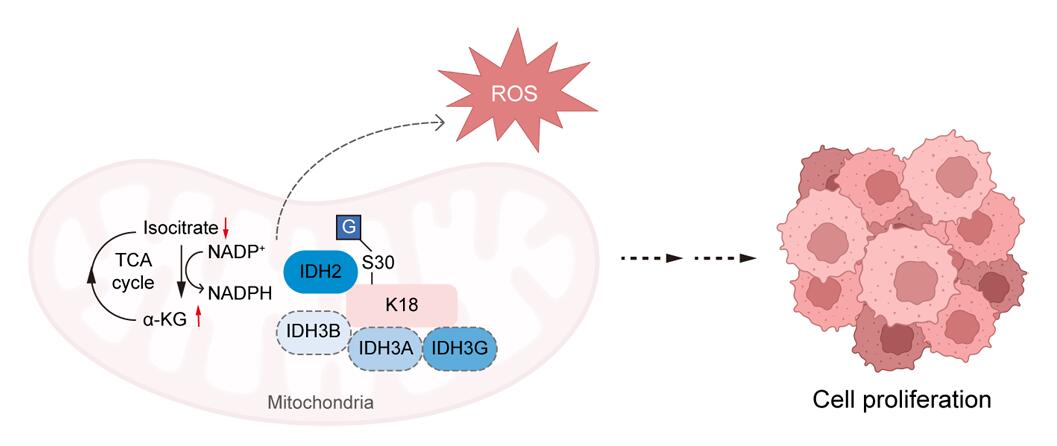
最后,研究者对K18的相互作用蛋白组进行了鉴定,并发现K18蛋白的Ser30位点的O-GlcNAc修饰促进了K18与异柠檬酸脱氢酶(isocitrate dehydrogenase, IDH)的相互作用,可能进一步参与调节胆管癌细胞的三羧酸(tricarboxylic acid, TCA)循环(图3)。
图3. O-GlcNAc修饰促进Keratin 18与IDH相互作用并调节胆管癌细胞的TCA 循环
综上,该研究利用化学糖蛋白组学Click-iG策略,对胆管癌中蛋白质O-GlcNAc修饰做了系统性的组学鉴定及分析,并揭示了K18蛋白Ser30位点的O-GlcNAc修饰可能通过促进K18与IDH的相互作用,进而调节胆管癌细胞的TCA循环,最终影响胆管癌的进展。这些发现为K18在胆管癌中的作用提供了新的机制见解,并提示了通过靶向Keratin 18蛋白的O-GlcNAc修饰治疗胆管癌的策略的潜在价值。
Proposed functional action of K18 O-GlcNAcylation in promoting CCA progression
摘要: Glycosylation plays a pivotal role in the intricate landscape of human cholangiocarcinoma (CCA), actively participating in key pathophysiological processes driving tumor progression. Among the various glycosylation modifications, O-linked β-N-acetyl-glucosamine modification (O-GlcNAcylation) emerges as a dynamic regulator influencing diverse tumor-associated biological activities. In this study, we employed a state-of-the-art chemical proteomic approach to analyze intact glycopeptides, unveiling the critical role of O-GlcNAcylation in orchestrating Keratin 18 (K18) and its interplay with tricarboxylic acid (TCA) cycle enzymes, specifically isocitrate dehydrogenases (IDHs), to propel CCA progression. Our findings shed light on the mechanistic intricacies of O-GlcNAcylation, revealing that site-specific modification of K18 on Ser 30 serves as a stabilizing factor, amplifying the expression of cell cycle checkpoints. This molecular event intricately fosters cell cycle progression and augments cellular growth in CCA. Notably, the interaction between O-GlcNAcylated K18 and IDHs orchestrates metabolic reprogramming by down-regulating citrate and isocitrate levels while elevating α-ketoglutarate (α-KG). These metabolic shifts further contribute to the overall tumorigenic potential of CCA. Our study thus expands the current understanding of protein O-GlcNAcylation and introduces a new layer of complexity to post-translational control over metabolism and tumorigenesis.
原文链接:https://pubs.acs.org/doi/10.1021/acscentsci.4c00163
DOI: https://doi.org/10.1021/acscentsci.4c00163
参考文献:[1] Banales, J. M. et al.Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020,17, 557-588.
[2] Silsirivanit, A.; Phoomak, C. & Wongkham, S. Glycosylation in cholangiocarcinoma development and metastasis: diagnostic and therapeutic considerations. In Diagnosis and Management of Cholangiocarcinoma, Ch. 25. Academic Press: Springer, 2021; pp 527-553.
[3] Moro, A. et al. The impact of preoperative CA19-9 and CEA on outcomes of patients with intrahepatic cholangiocarcinoma. Ann. Surg. Oncol. 2020, 27, 2888-2901.
[4] Silsirivanit, A. Glycans: potential therapeutic targets for cholangiocarcinoma and their therapeutic and diagnostic implications. Expert Opin. Ther. Targets. 2021, 25, 1-4.
[5] Silsirivanit, A. et al.Multi-serum glycobiomarkers improves the diagnosis and prognostic prediction of cholangiocarcinoma. Clin. Chim. Acta. 2020, 510, 142-149.
[6] Ament, C. E. et al.Aberrant fucosylation sustains the NOTCH and EGFR/NF-kB pathways and has a prognostic value in human intrahepatic cholangiocarcinoma. Hepatology. 2023, 78, 1742-1754.
[7] Park, D. D. et al. Metastasis of cholangiocarcinoma is promoted by extended high-mannose glycans. Proc. Natl. Acad. Sci. 2020, 117, 7633-7644.
[8] Wattanavises, S. et al.Increase of MAL-II binding alpha2,3-sialylated glycan is associated with 5-FU resistance and short survival of cholangiocarcinoma patients. Medicina. 2019, 55, 761.
[9] Hart, G. W., Housley, M. P. & Slawson, C. Cycling of O-linked β-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007, 446, 1017-1022.
https://m.sciencenet.cn/blog-446272-1431449.html
上一篇:癌症意味着什么?What is the meaning of cancer?
下一篇:姜长涛教授学术讲座:肠道菌源酶跨物种调控宿主代谢性疾病